A deeper understanding of the brain’s connectivity network of neurons and its relationship to the organ’s deep tissue could allow researchers to predict brain spatial patterns and recognize what processes relate to neurological disorders, according to a new study from Weill Cornell Medicine and UC at San Francisco.
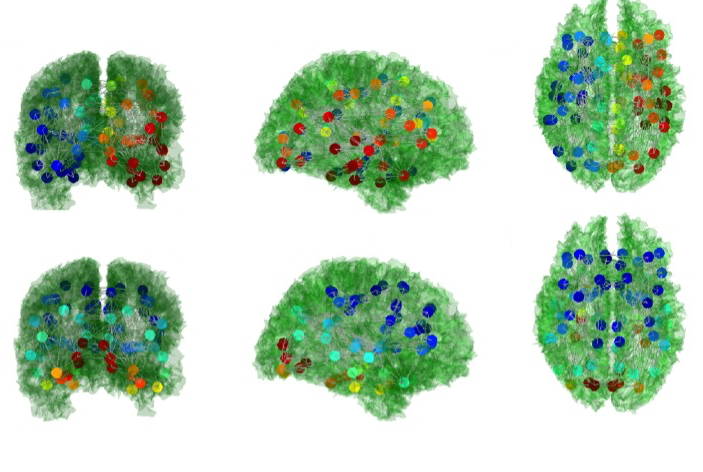
In their study, published June 22 in PLoS Computational Biology, the investigators used mathematics and a form of magnetic resonance imaging called Diffusion-Tensor MRI to better understand how neurological disorders affect the connections between the brain’s deep white matter and its network of fibers, whose connectivity patterns are called the structural connectome. They discovered sub-networks that make up the connectome, called eigenmodes, which have a unique role in communicating information from one region of the brain to another.
“Once you understand what the eigenmodes look like, you can then start to understand what processes occur in the brain and what patterns of activity to expect,” said senior author Dr. Ashish Raj, an associate professor of computer science in radiology and of neuroscience in the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine.
Conventional wisdom suggests that each region of the brain plays a specific functional role — from motor coordination to the creativity and artistic expression requisite for poetry — and damage to these regions affects patients accordingly. But recent studies have eschewed that philosophy, finding instead that the brain is more than the sum of its parts.
Dr. Raj described the brain’s connectome as a series of pipes through which information flows. The connectome’s constituent parts — its eigenmodes — are “similar to the vibrations on a guitar,” he said. The researchers performed MRIs on 10 healthy subjects and found that these eigenmodes are ubiquitous with high overlap between subjects and between scans of the same subject performed on different days.
“The eigenmodes wouldn’t be useful if they weren’t consistent, so this was an important finding,” Dr. Raj said.
Dr. Raj and his team then investigated what part of the brain’s white matter region “acts as an anchor” for the eigenmodes by virtually simulating lesions, which would damage the brain and disrupt network connections. They determined where lesions were most critical in terms of how they affected the eigenmodes and found that the most neurologically important areas of the brain — the parts which produced the highest change in eigenmodes — were at the center of white matter fiber bundles.
The researchers also studied how eigenmodes might change in impaired brains. They investigated a neurodevelopmental disorder called Agenesis of the corpus callosum (AgCC), where the corpus callosum, which connects the left and right hemispheres of the brain, is absent.
“Normally if two halves of a brain do not talk to each other, the brain will not be able to function properly. But it turns out most people with Agenesis of the corpus callosum don’t have tremendously impaired brains,” Dr. Raj said.
To determine why this was the case, the researchers studied the eigenmodes in brains with AgCC and found that they were not significantly different from healthy controls. Instead it appeared that some of the network connections in brains with AgCC regenerated from an alternative pathway, which compensated for the lack of corpus callosum.
“We expected their eigenmode structure to be completely alien compared to healthy controls,” Dr. Raj said. “That was the biggest surprise of the paper.”
Dr. Raj plans on studying eigenmodes further with the hope that they can soon act as biomarkers for disease. “If the structure of the eigenmode is changed by disease, then they can be used to measure the effects of disease,” he said.

